Recommendations for asthma monitoring in children: A PeARL document endorsed by APAPARI, EAACI, INTERASMA, REG, and WAO
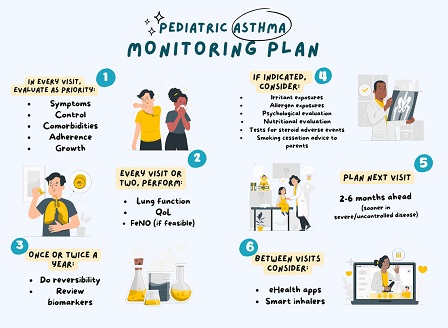
Monitoring is a major component of asthma management in children. Regular monitoring allows for diagnosis confirmation, treatment optimisation, and natural history review. Numerous factors that may affect disease activity and patient well-being need to be monitored:
- Response and adherence to treatment
- Disease control
- Disease progression
- Comorbidities
- Quality of life
- Medication side-effects
- Allergen and irritant exposures
- Diet and more.
However, the prioritisation of such factors and the selection of relevant assessment tools is an unmet need. Furthermore, rapidly developing technologies promise new opportunities for closer, or even “real-time” monitoring between visits. Following an approach that included needs assessment, evidence appraisal, and Delphi consensus, the PeARL Think Tank, in collaboration with major international professional and patient organisations, has developed a set of 24 recommendations on pediatric asthma monitoring, to support healthcare professionals in decision-making and care pathway design.
Pediatric Asthma in Real Life (PeARL) is a think tank set up by the Respiratory Effectiveness Group consisting of health care professionals, clinical academics, and expert patient representatives with expertise in and professional exposure to paediatric asthma. Asthma monitoring was prioritized in a previous PeARL report evaluating unaddressed clinical needs in pediatric asthma
Link to the full article:
https://doi.org/10.1111/pai.14129